We are developing tecarfarin which is designed to be a superior and safer vitamin K antagonist (VKA), to elevate care for underserved patients: initial priorities are those with implanted medical devices such as left ventricular assist devices (LVADs) and those with end-stage kidney disease (ESKD) plus atrial fibrillation (AFib).
Tecarfarin is a Phase 3-ready, vitamin K antagonist (VKA), taken once a day as an oral anticoagulant like warfarin. Tecarfarin, like warfarin, was designed to have the same well-established and reversible VKA mechanism of action, but to be free of certain potentially life-threatening clearance and drug-to-drug interaction problems and the cumbersome management associated with warfarin.
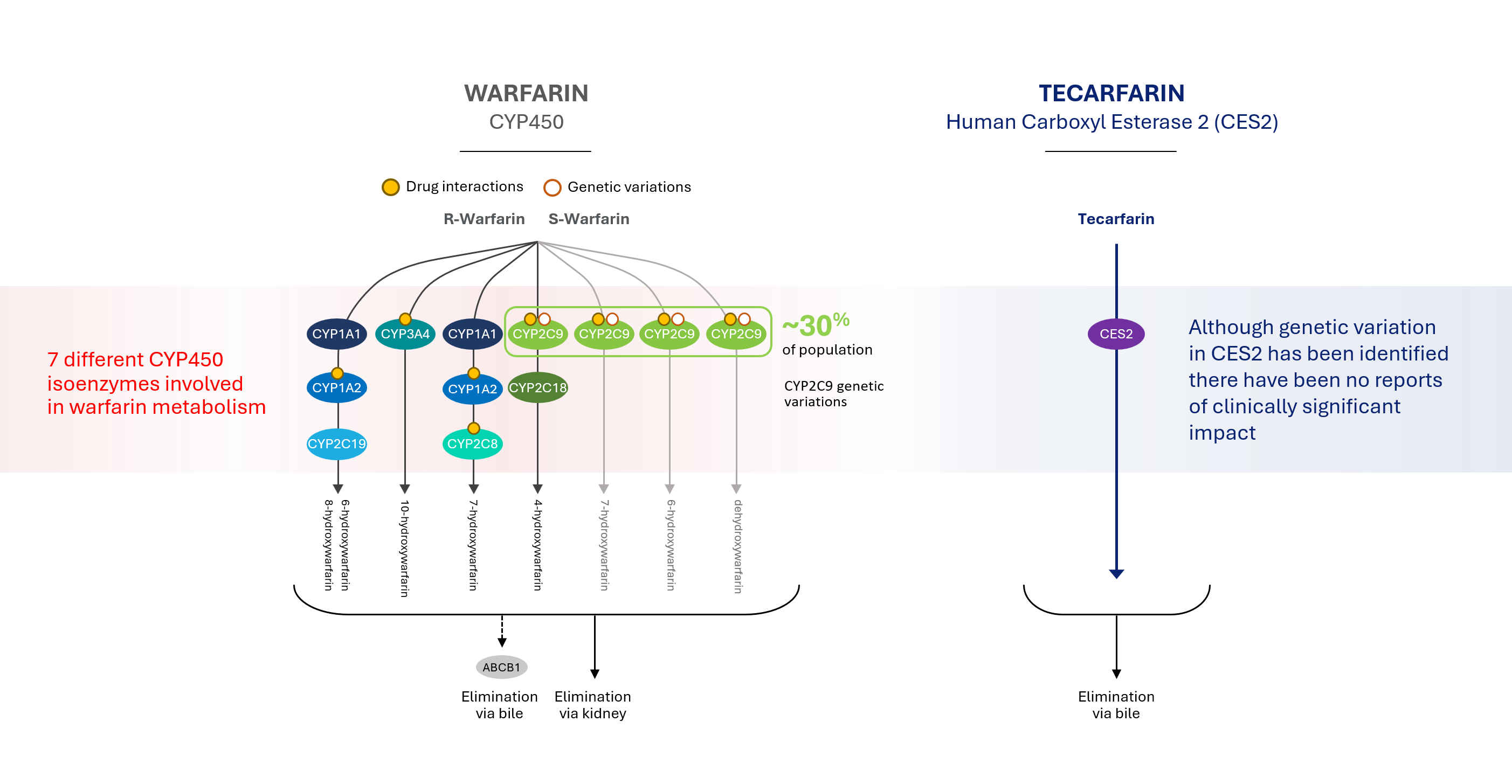
Tecarfarin is metabolized via a different pathway, one that is abundant and essentially unsaturable, thereby avoiding the bottleneck in the CYP450 pathway where warfarin is metabolized. Warfarin metabolism is complicated by known competitors, inhibitors and inducers as well as the established impact of genetic variants. In contrast, tecarfarin is metabolized via the human carboxyl esterase 2 pathway (CES2), providing more effective, safe and consistent anticoagulation versus warfarin.
The link between heart failure and eskd
Renal dysfunction is a common comorbidity in patients with advanced heart failure who may benefit from mechanical circulatory support. Unfortunately, renal dysfunction may result after LVAD implantation. It has been reported that renal dysfunction is associated with a reduced survival rate after LVAD implantation, especially in patients with ESKD at the time of implantation.
The adverse effects of LVAD implantation, such as infections, bleeding, strokes, renal failure, and right heart failure, are known to result in poor outcomes. LVADs are found to have an acceptable survival for carefully selected patients on dialysis. Even so, this still requires multidisciplinary approaches and shared decision-making. As of today, there have been limited studies regarding which hemodynamic strategy is most effective in this patient population and if such an approach may improve survival rates with the current advances in left ventricular assist devices.
Tecarfarin has proven to have a better pharmacokinetic profile than warfarin in chronic kidney disease patients.
Advancing Tecarfarin
Tecarfarin received orphan drug designation for heart failure patients with left ventricular assist devices (LVADs) as well as both orphan drug and fast track status for end-stage kidney disease patients (ESKD) with atrial fibrillation (Afib).
We believe tecarfarin has the potential to be a safer, more effective anticoagulation for patients with these rare medical conditions.
Proven Safety Profile
Tecarfarin has been evaluated in 11 human clinical trials in more than 1,000 individuals. In Phase 1, Phase 2 and Phase 2/3 clinical trials that have conducted thus far, tecarfarin has generally been well-tolerated in both healthy adult patients and patients with chronic kidney disease.
Improved Metabolic Profile
Drug metabolism refers to the process by which a drug is inactivated by the body and rendered easier to eliminate or to be cleared by the body. Most approved drugs which are prescribed for the treatment of thrombosis, are metabolized in the liver through a pathway known as the Cytochrome CYP450 system, or CYP450, by the enzymes known as CYP2C9 and CYP3A4. By using a different metabolic pathway, tecarfarin eliminates or minimizes the CYP450 metabolism in the liver.
Patients taking multiple medications, such as those with implanted cardiovascular medical devices, and patients with impaired kidney function can experience an overload in the pathway, creating a bottleneck that often leads to insufficient clearance, which results in a toxic build-up of one or more drugs. In some instances, patients taking multiple medications metabolized by the same CYP450 pathway may experience decreased efficacy of one or more of the medications due to rapid metabolism or increased drug effect and/or toxicity due to enzyme induction.
Our product candidate tecarfarin was designed to follow a metabolic pathway distinct from the CYP450 pathway and is metabolized by both CYP450 and non-CYP450 pathways. We believe this may allow elimination by large capacity and non-saturable tissue esterase pathways that exist throughout the body rather than just in the liver.
Tecarfarin’s Metabolic Advantage
Tecarfarin is metabolized via an alternate pathway that is abundant and essentially insaturable, thereby avoiding the bottleneck in the CYP450 pathway where warfarin in metabolized.