Tecarfarin
Tecarfarin is a Phase 3-ready vitamin K antagonist (VKA) taken once daily as an oral anticoagulant, similar to warfarin.
Tecarfarin has been evaluated in eleven (11) human clinical trials in over 1,000 individuals. In Phase 1, Phase 2 and Phase 2/3 clinical trials, tecarfarin has generally been well-tolerated in both healthy adult subjects and in patients with chronic kidney disease.
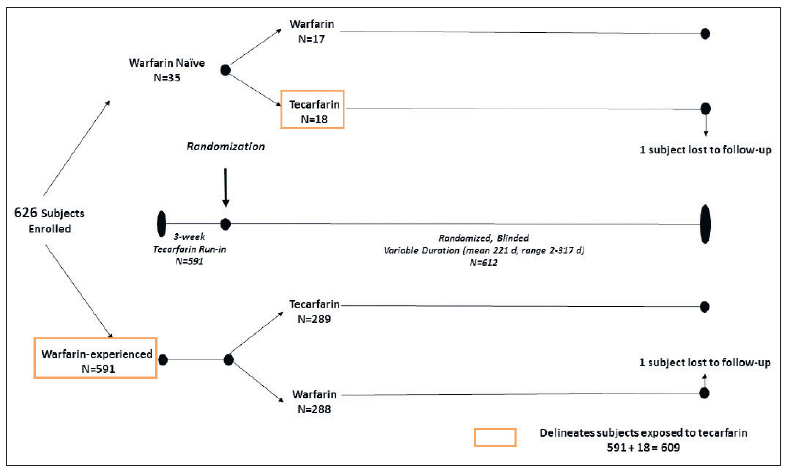
The Phase 2/3 EMBRACE-AC study was a Phase 2/3 trial multi-center, randomized, double-blind, parallel group, active control trial that compared tecarfarin to warfarin in 607 patients with indications for chronic anticoagulation, with a primary endpoint of time in therapeutic range (TTR).
Whitlock RP et al. Thromb Haemost 2016; 116(02): 241-250)
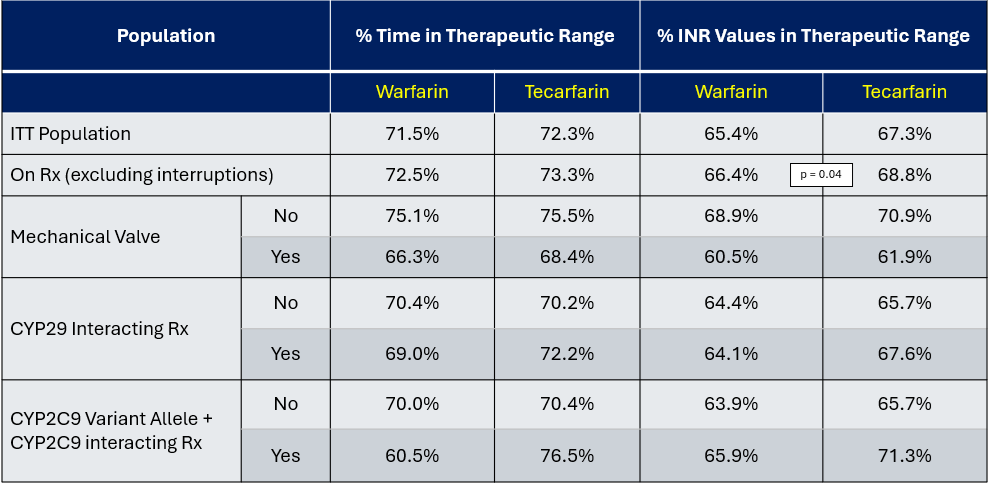
Dosing of study drugs was managed by a centralized dose control center. As a result of this aggressive management, the TTRs in this study were higher than in general practice. A stable dose of tecarfarin (<10 % variation in weekly dose for 3 consecutive weeks with the INR within the therapeutic range) was attained in 94.5 % of the tecarfarin patients. The mean TTR with tecarfarin (the primary endpoint) was numerically higher but not significantly superior to warfarin in terms of the primary endpoint (72.3% with tecarfarin vs 71.5% with warfarin; p=0.51); numerically virtually all subgroups favored tecarfarin, which was especially noteworthy given the aggressiveness of INR management in the warfarin group. EMBRACE-AC also included analyses of INR measurements while patients were temporarily off their trial drug for other medical reasons (like bleeding). In a post-hoc analysis excluding INR values while off therapy, the number of INR values in the therapeutic range was significantly higher on tecarfarin (68.8%) than on warfarin (66.4%) (p<0.04).
% TTR and % INR Values in therapeutic Range in EMBRACE AC
Tecarfarin was well tolerated – only 1.6% of the tecarfarin subjects had major bleeding and there were no thrombotic events.
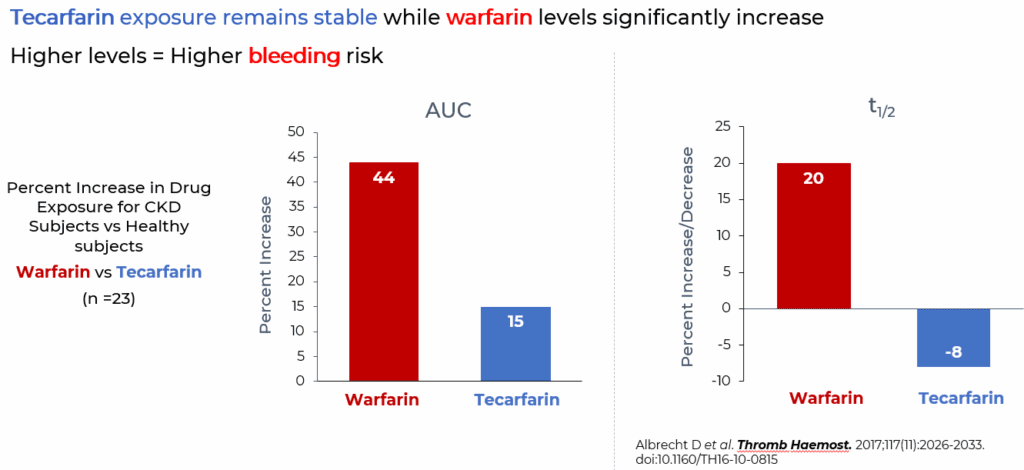
In a subsequent Phase 1 single-dose study comparing 13 patients with Stage 4 CKD and 10 healthy volunteers, the metabolism of warfarin was shown to be significantly inhibited, whereas tecarfarin metabolism was not altered, in the setting of significant renal insufficiency.