TECARFARIN FOR CHRONIC ANTICOAGULATION
With the only novel VKA blood thinner currently in development, Cadrenal is challenging the status quo to provide better care for patients with conditions for which current treatments are ineffective or suboptimal.
Our lead product candidate, tecarfarin, is a novel late-stage, reversible oral VKA in the same drug class as warfarin, designed to prevent heart attacks, strokes, and deaths due to blood clots in patients requiring chronic anticoagulation.
The present development of tecarfarin is focused on two orphan cardiovascular conditions with particularly narrow therapeutic windows, where patients may be unable to achieve sufficiently reliable chronic anticoagulation with warfarin, and where DOACs have either failed or their efficacy and safety remain unproven: ESKD (end-stage kidney disease) patients with atrial fibrillation, and patients with implanted LVADs (left ventricular assist devices).
Advancing Toward Approval
Regulatory Designations Highlight Unmet Need and Clinical Promise
Tecarfarin received Orphan Drug Designation (ODD) and fast-track designation from USFDA for the prevention of systemic thromboembolism (blood clots) of cardiac origin in ESKD patients with AFib.
Tecarfarin also received ODD from USFDA for the prevention of thromboembolism and thrombosis in patients with implanted mechanical circulatory support devices, including LVADs.
PROVEN SAFETY PROFILE
Extensive clinical evaluation underscores Tecarfarin’s favorable safety and tolerability.
Tecarfarin has been evaluated in 11 human clinical trials in more than 1,000 individuals. In Phase 1, Phase 2 and Phase 2/3 clinical trials that have conducted thus far, tecarfarin has generally been well-tolerated in both healthy adult patients and patients with chronic kidney disease.
IMPROVED METABOLIC PROFILE
Engineered for improved metabolic stability and fewer complications in patients with kidney impairment or on multiple medications.
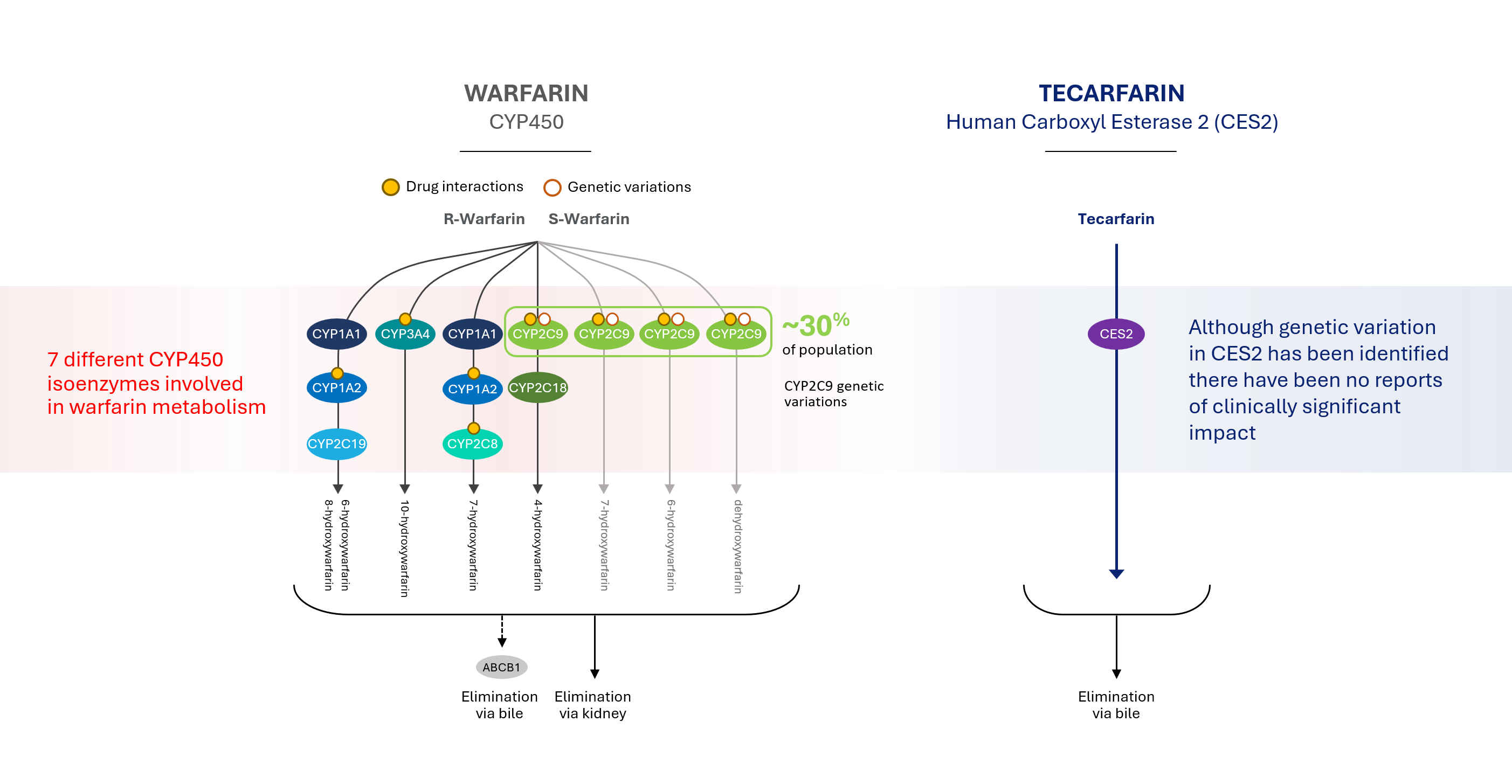
Drug metabolism refers to the process by which a drug is inactivated by the body and rendered easier to eliminate or to be cleared by the body. Most approved drugs that are prescribed for the treatment of thrombosis are metabolized in the liver through a pathway known as the Cytochrome CYP450 system, or CYP450, by the enzymes known as CYP2C9 and CYP3A4. By using a different metabolic pathway, tecarfarin eliminates or minimizes the CYP450 metabolism in the liver.
Patients taking multiple medications, such as those with implanted cardiovascular medical devices, and patients with impaired kidney function can experience an overload in the pathway, creating a bottleneck that often leads to insufficient clearance, which results in a toxic build-up of one or more drugs. In some instances, patients taking multiple medications metabolized by the same CYP450 pathway may experience decreased efficacy of one or more of the medications due to rapid metabolism or increased drug effect and/or toxicity due to enzyme induction.
Our product candidate tecarfarin was designed to follow a metabolic pathway distinct from the CYP450 pathway and is metabolized by both CYP450 and non-CYP450 pathways. We believe this may allow elimination by large capacity and non-saturable tissue esterase pathways that exist throughout the body rather than just in the liver.